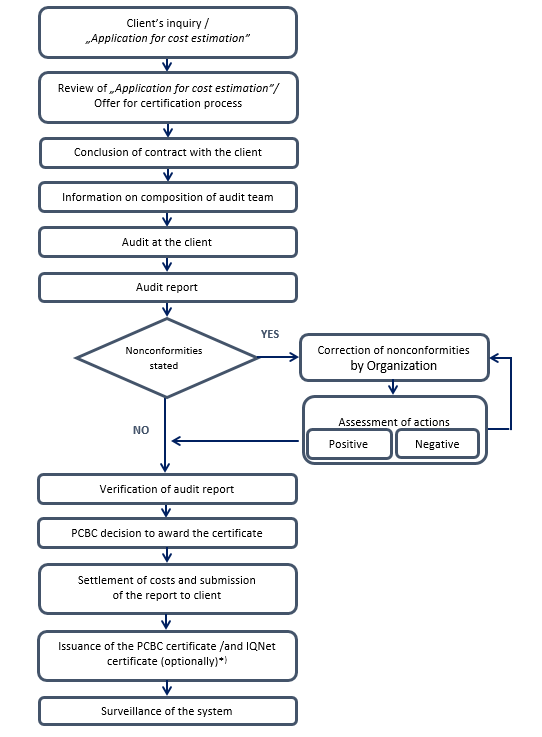
Description of system
Medical Products. Quality Management Systems. Requirements for regulatory purposes
The ISO 13485 international standard includes requirements referring to management system and is intended for organizations willing to evidence the ability to provide medical products and related services. The basic objective of the standard is to facilitate harmonizing the requirements of regulations concerning medical products with quality management systems.